Please refer to the Special Warnings and Precautions for use (section 4.4) of the Summary of Product Characteristics for full information.
Methylphenidate treatment is not indicated in all children with ADHD and the decision to use the drug must be based on a very thorough assessment of the severity and chronicity of the child’s symptoms in relation to the child’s age.1
Methylphenidate treatment is usually discontinued during or after puberty.1
Patients on long-term therapy (i.e. over 12 months) must have careful ongoing monitoring for cardiovascular status, growth, appetite, development of de novo or worsening of pre-existing psychiatric disorders.1
Patients who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden cardiac or unexplained death or malignant arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further specialist cardiac evaluation if initial findings suggest such history or disease.1
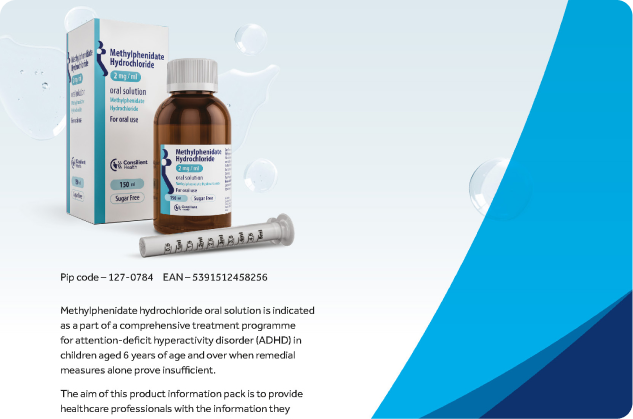
Indications and Patient Selection
Methylphenidate is not for all children with ADHD. Treatment decisions should consider symptom severity and chronicity. Long-term use (over 12 months) lacks sufficient controlled trial data and requires periodic reassessment, especially during puberty. Discontinuation during school holidays is recommended to evaluate the child’s progress without medication.
Usage in Specific Age Groups
Adults: Not approved for adult ADHD treatment; safety and efficacy are unconfirmed.
Elderly: Not recommended; safety and efficacy data are lacking.
Children under 6: Should not be used due to a lack of safety and efficacy data.
Cardiovascular Considerations
A detailed cardiac history and assessment are mandatory, especially for those with personal or family history of heart issues. Patients should be monitored for blood pressure and pulse changes at each dose adjustment and every six months thereafter. Methylphenidate is contraindicated for individuals with specific cardiovascular conditions due to risks like sudden death and adverse cardiac events.
Please refer to the Contraindications section below for further information. For Cerebrovascular disorders, please refer to the Summary of Product Characteristics and the Contraindications section below.
Psychiatric Precautions
Psychiatric disorders are common in ADHD and may worsen with methylphenidate. Development or worsening or psychiatric disorders should be monitored at every dose adjustment, then at least every 6 months, and at every visit: discontinuation of treatment may be appropriate. Patients should be closely monitored for:
Psychosis or mania: Can worsen; discontinuation may be necessary.
Aggression and hostility: May emerge or worsen; regular monitoring advised.
Suicidal ideation: Any emergence should prompt immediate evaluation.
Tics and Tourette’s syndrome: May worsen; family history should be evaluated.
Anxiety, agitation, and bipolar disorder: Can worsen; detailed psychiatric history is required.
Other Risks
Growth and Weight: Growth retardation has been reported. Monitor height, weight, and appetite every six months.
Seizures: Caution in epilepsy patients; discontinue if seizure frequency increases.
Abuse Potential: Methylphenidate has a risk of misuse and dependency. Monitor especially those with past substance abuse or co-morbid behavioural disorders.
Withdrawal: May reveal underlying depression or chronic hyperactivity; careful supervision is advised.
Fatigue: Should not be used for general fatigue prevention or in cases with a risk of misuse.
Please refer to the Special Warnings and Precautions for use (section 4.4) of the Summary of Product Characteristics for full information.